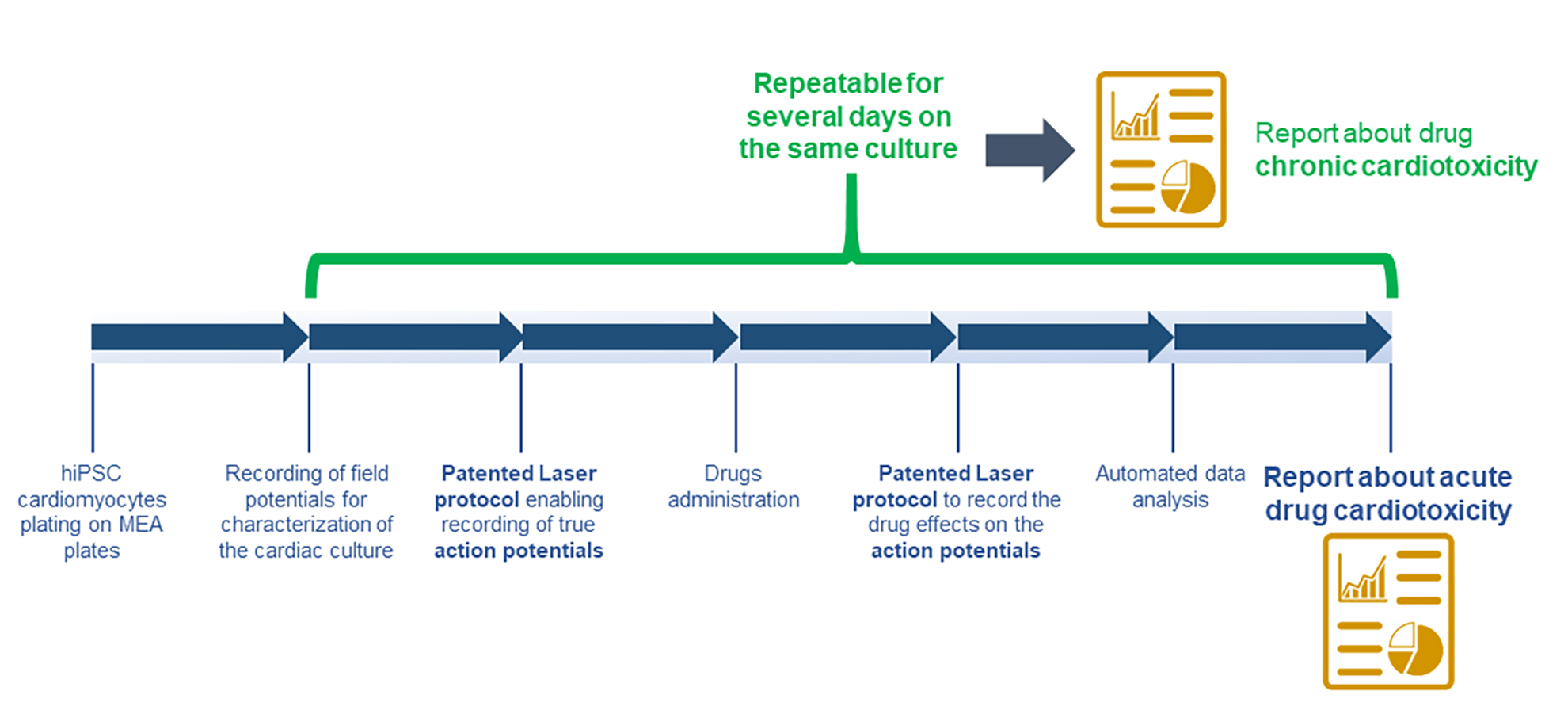
Drug toxicity, manifested also as cardiotoxicity, often generates drug attrition. Therefore, an early-stage detection of potential toxicity in drug discovery can save both time and developmental costs, and, most importantly, reduce the late-stage failure and potential withdrawn.
Foresee biosystems exploits innovative solutions to assist you in understanding the toxic liability of your compounds, identifying which of them have the optimal safety profile to advance into the clinic. Our focus on LASER technology and automation allow for high quality data, generated rapidly and cost-effectively. To that end, Foresee biosystems provides you a service based on our LASER scanning technique to simultaneously measure cardiac action potential as indicators of cardiotoxicity, thus improving the prediction of toxicological effects on in-vitro cardiac cells model.
We offer an on-demand service to evaluate cardiotoxicity in human iPS cell-derived cardiomyocytes. Using microelectrode array and recording field potential, we determine whole cell electrophysiology measuring beat rate, field potential duration, amplitude and conduction velocity. Then, using the same microelectrode array coupled with Foresee technology, we provide the most precise and reliable in vitro assay, recording intracellular action potential that, different from the field potential, contains information about ions channels activity in the cell membrane, for the best assessment of cardiotoxicity actual on the market. Given the very low level of invasiveness of our technology, the Viability is maintained for an extended culture periods (up to 5 weeks) allowing for acute and chronic studies.
You will benefit from
Our service
Example 1: Effects of E-4031 on intracellular action potentials recorded from cardiomyocytes
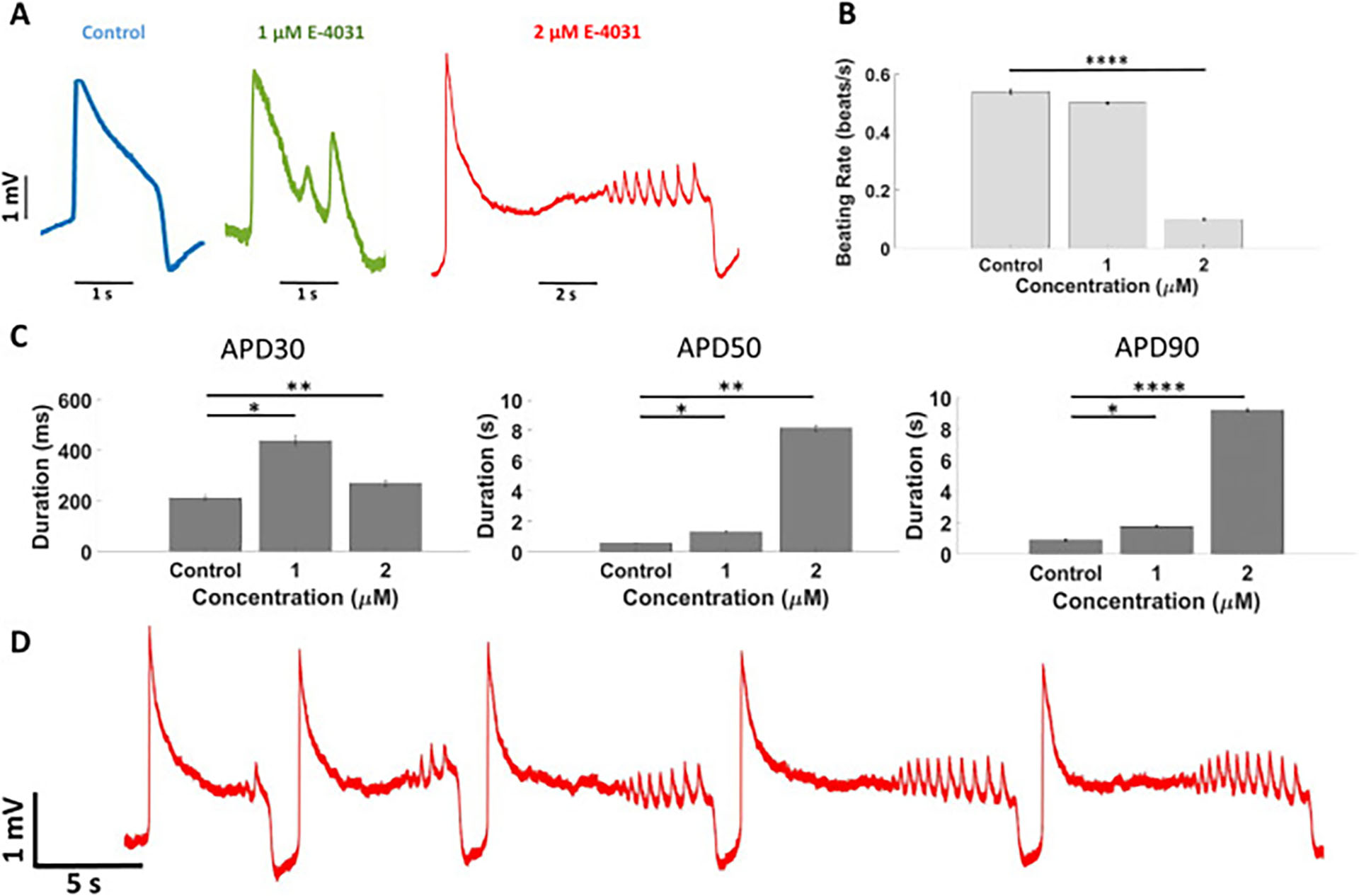
(A)Action potential waveforms in control conditions and at tested concentrations of E-4031, which show clear early afterdepolarizations (EADs). (B)Beating rate before (Control) and after E-4031 administration. (C) ADP at different amplitudes (APD30, 50, 90). (D) Temporal dynamic evolution of APs after administration of 2 μM E-4031. Data are represented as mean ± SEM. N = 70 (Control and 1 μM), N = 95 (2 μM).
Iachetta, G., Colistra, N., Melle, G., Deleye, L., Tantussi, F., De Angelis, F., & Dipalo, M. (2021). Improving reliability and reducing costs of cardiotoxicity assessments using laser-induced cell poration on microelectrode arrays. Toxicology and Applied Pharmacology.
Example 2: dose-dependent effects of Quinidine on intracellular action potentials recorded from Pluricyte and iCell cardiomyocytes.

(A, Band C)Pluricyte action potential waveforms, beating rate and APD at different amplitude (APD30, 50, 90). (D, E and F) iCell action potential waveforms with early afterdepolarizations (EADs), beating rate and APD at different amplitude (APD30, 50, 90). Data are represented as mean ± SEM. N = 40 (Control), N = 50 (1 μM), N = 50 (2 μM), N = 6 (10 μM) for Pluricyte. N = 64 (Control), N = 48 (1 μM), N = 46 (2 μM), N = 35 (10 μM) for iCell.
Iachetta, G., Colistra, N., Melle, G., Deleye, L., Tantussi, F., De Angelis, F., & Dipalo, M. (2021). Improving reliability and reducing costs of cardiotoxicity assessments using laser-induced cell poration on microelectrode arrays. Toxicology and Applied Pharmacology.
Example 3: dose and time dependent effects of Pentamidine on intracellular action potentials.
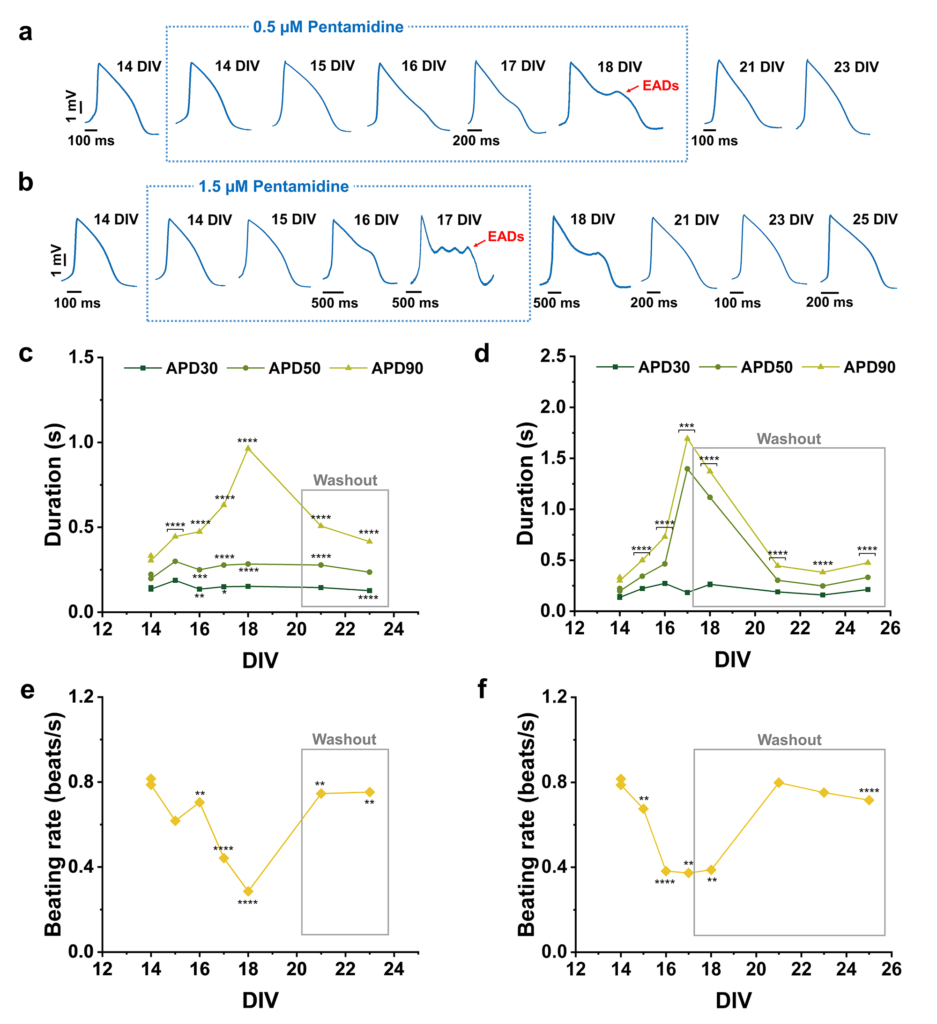
Long-term effect of pentamidine on hiPSC-CMs. (A and B) Action potential mean waveforms at different days after 0.5 µM and 1.5 µM pentamidine administration, respectively. (C and D) Action potential duration (APD) at different amplitudes after 0.5 µM and 1.5 µM pentamidine administration, respectively. (E and F) Beating rate after 0.5 µM and 1.5 µM pentamidine administration, respectively.
, , , , ,
FILL OUT THE FORM
