Experience the Power: Click to Discover Our Technology’s Applications
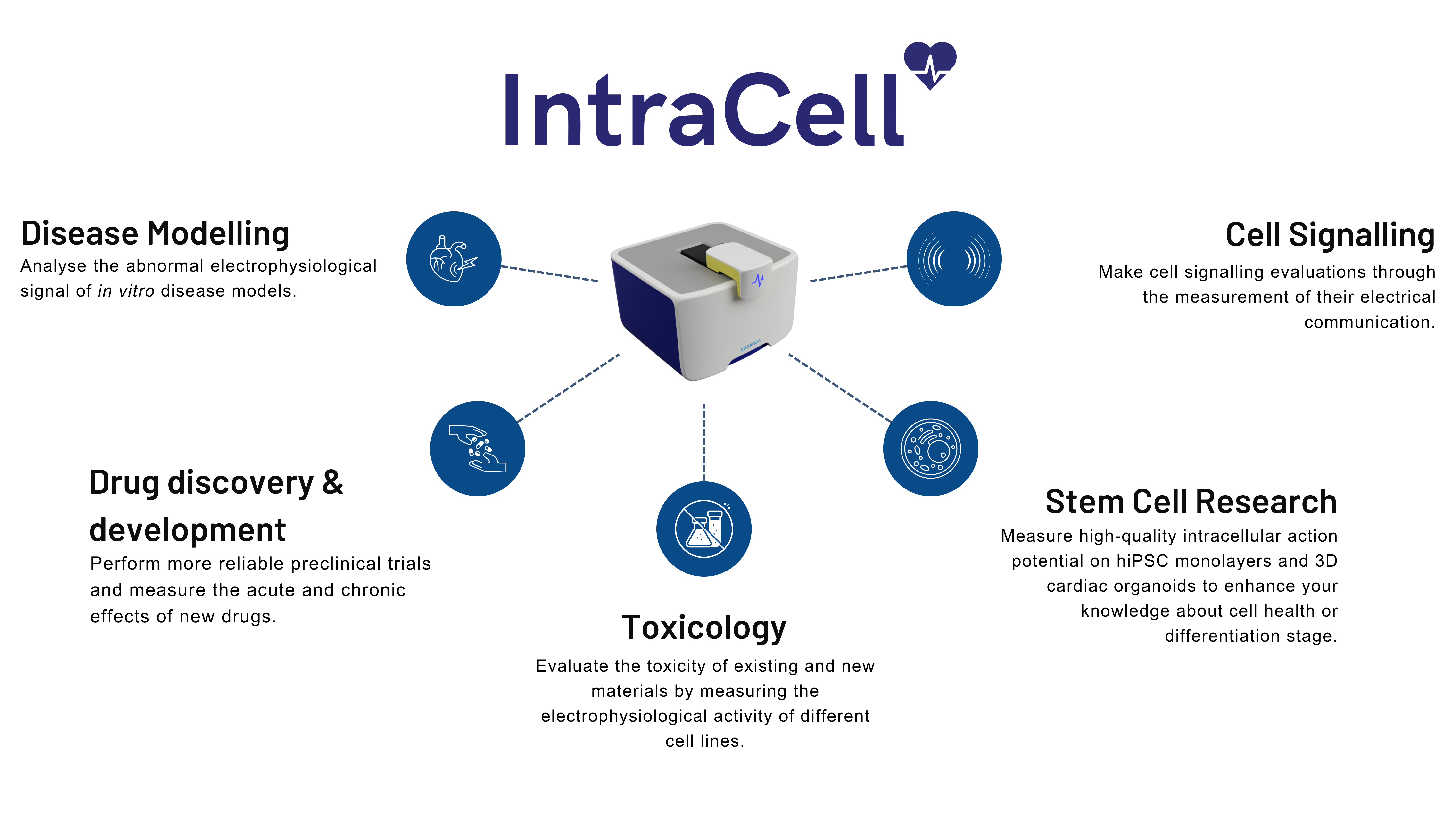
Cardiac drug safety is a major concern and one of the sticking points throughout cardiac drug development. Therefore, improving in vitro assays at the early phases of drug development is critical in preventing late-stage failure.
In vitro cell cultures are a powerful models for the accurate analysis of human heart disorders and therefore suitable to perform cardiotoxicity assays. Many of these disorders are the result of subtle changes to cardiomyocyte excitability, contractility, or both. Therefore, an early-stage detection of potential toxicity in drug discovery can save both time and developmental costs, and, most importantly, reduce the late-stage failure and potential withdrawn. Our products aim to provide our customers with powerful tool for accurately measuring changes in depolarization, repolarization, and, indeed, detecting drug-related adverse responses.
FILL OUT THE FORM